Geistlich Fibro-Gide®
User Benefits
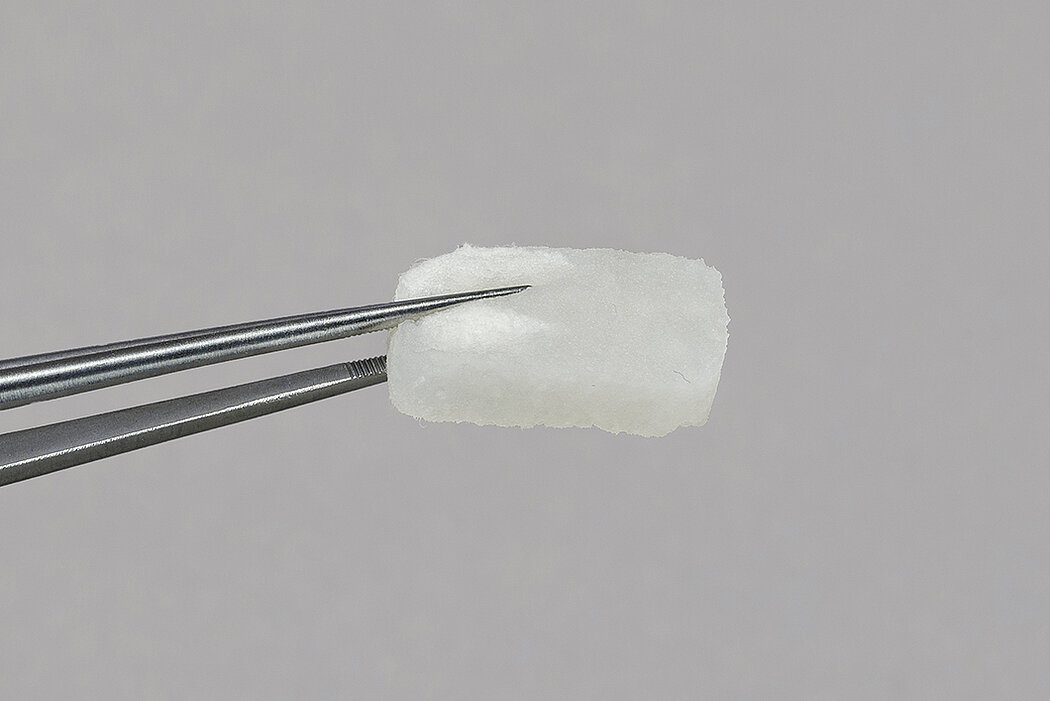
Geistlich Fibro-Gide® is a porcine, porous, resorbable and volume-stable collagen matrix, specifically designed for soft-tissue regeneration.1,4,10 The collagen matrix is made of reconstituted collagen and undergoes smart chemically cross-linking to improve its volume stability while maintaining a good biocompatibility.1-3,4-10 The porous network of Geistlich Fibro-Gide® supports angiogenesis, formation of new connective tissue and stability of the collagen network in submerged healing.2,3 In vivo animal models have shown good integration of Geistlich Fibro-Gide® into the surrounding soft-tissue while maintaining stability.4
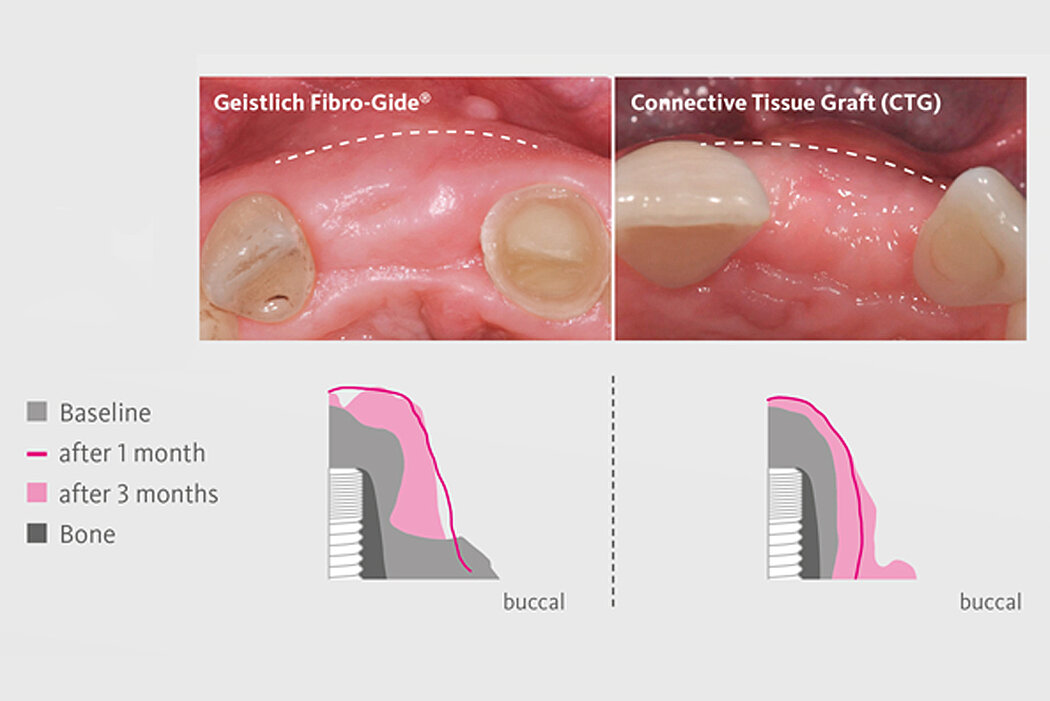
Geistlich Fibro-Gide® is the alternative to autogenous connective tissue grafts (CTG), which are considered as the gold standard in regenerative soft-tissue procedures.3,5,6 With Geistlich Fibro-Gide® additional harvest site is avoided, patient morbidity is reduced.3,7-8
References
- Mathes SH. Et al. Biotechnol Bioeng. 2010 Dec 15;107(6):1029-39. (preclinical study).
- Thoma DS. et al. Clin Oral Implants Res. 2015 Mar; 26(3): 263–70. (preclinical study)
- Thoma DS. et al. J Clin Periodontol. 2016 Oct; 43(10): 874–85. (clinical study)
- Thoma DS. et al. Clin Oral Implants Res. 2012 Dec; 23(12): 1333–9. (preclinical study)
- Thoma DS. et al. Clin Oral Implants Res. 2009 Sep; 20 Suppl 4: 146–65. (systematic review based on clinical studies)
- Thoma DS. et al. J Clin Periodontol. 2014 Apr; 41 Suppl 15: S77–91. (systematic review based on clinical studies)
- Del Pizzo M. et al. J Clin Periodontol. 2002 Sep; 29(9): 848–54. (clinical study)
- Soileau KM. & Brannon RB. J Periodontol. 2006 Jul; 77(7): 1267–73. (clinical study)
- Zeltner M. et al. J Clin Periodontol. 2017 Apr; 44(4): 446–453. (clinical study)
- Data on file. Geistlich Pharma AG, Wolhusen, Switzerland.
Geistlich Fibro-Gide®
Inspired by Nature, engineered by Geistlich
The collagen expertise of Geistlich has led to a tailor-made product meeting the clinical needs and providing therapeutic solutions in oral soft-tissue regeneration. More than 1,000 prototypes were developed and tested in order to get a product like Geistlich Fibro-Gide®.10
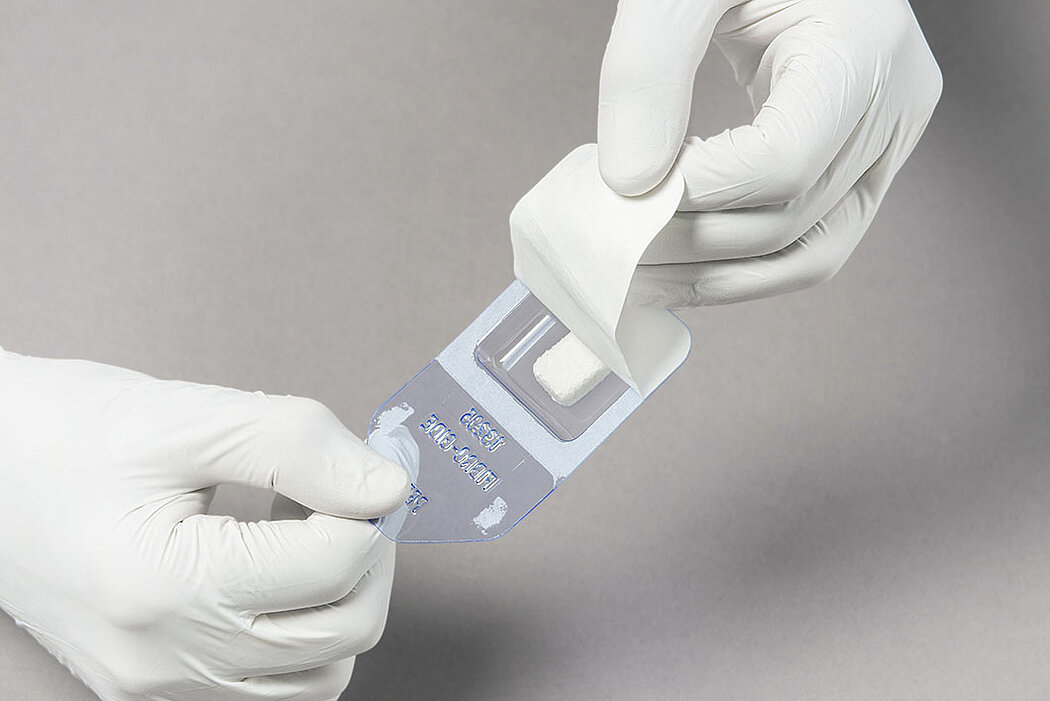
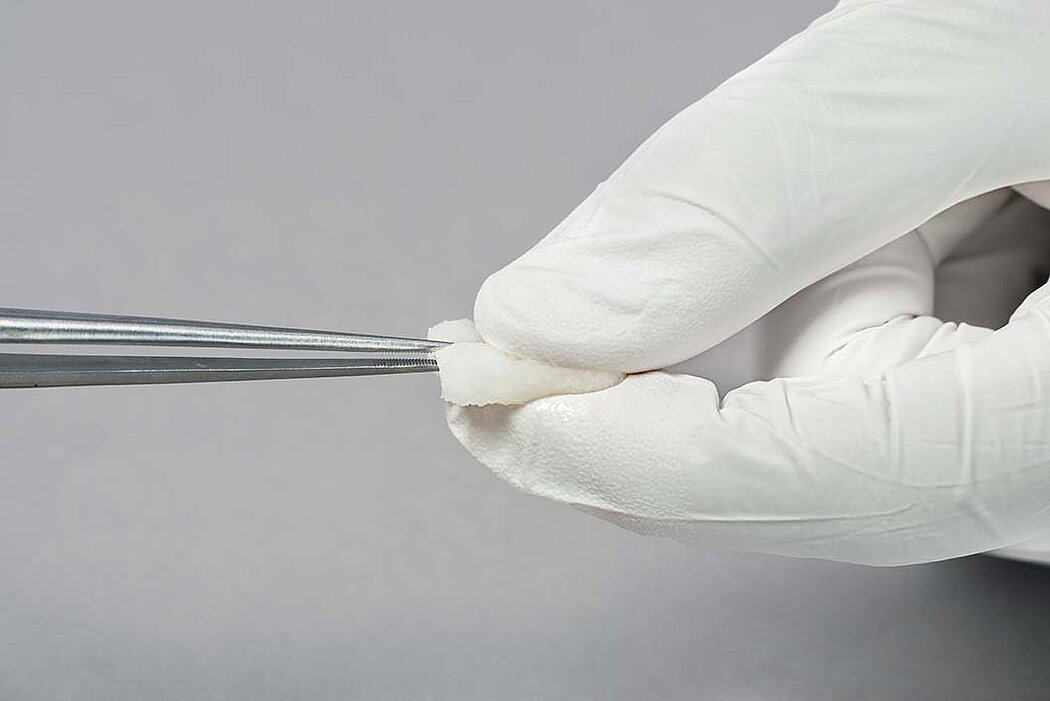
Handling of Geistlich Fibro-Gide® is very easy compared to connective tissue grafts:
- Unlimited availability and consistent quality.1
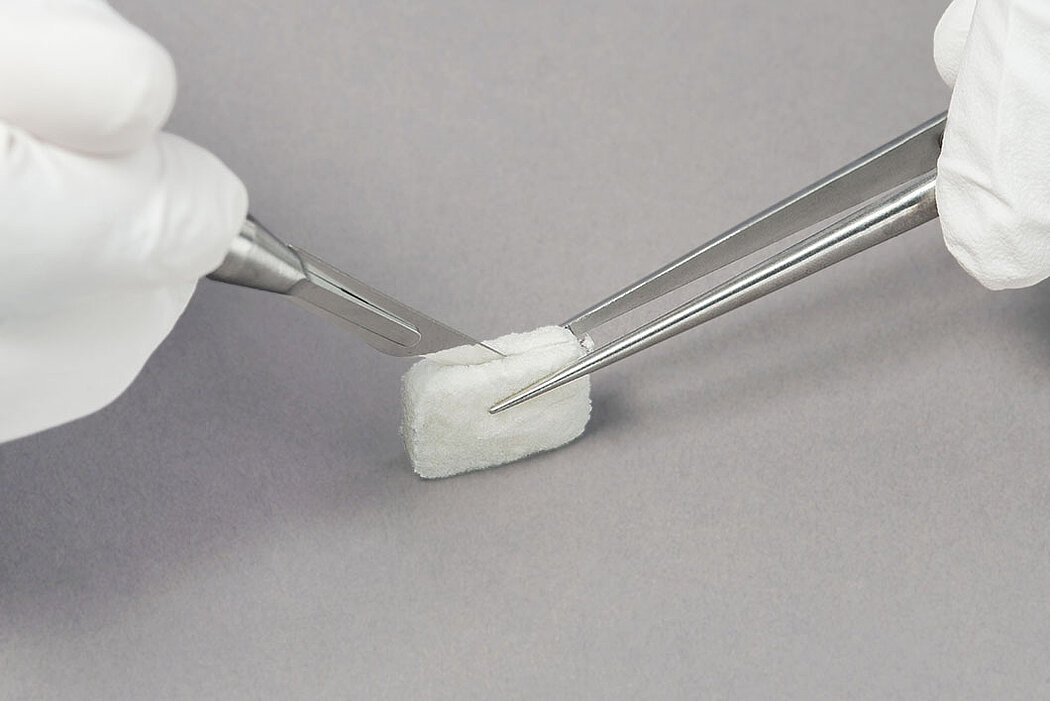
- Measure the defect and trim the matrix to the desired size and thickness.1
- Once moist, the matrix can be further trimmed to the desired size and thickness.1
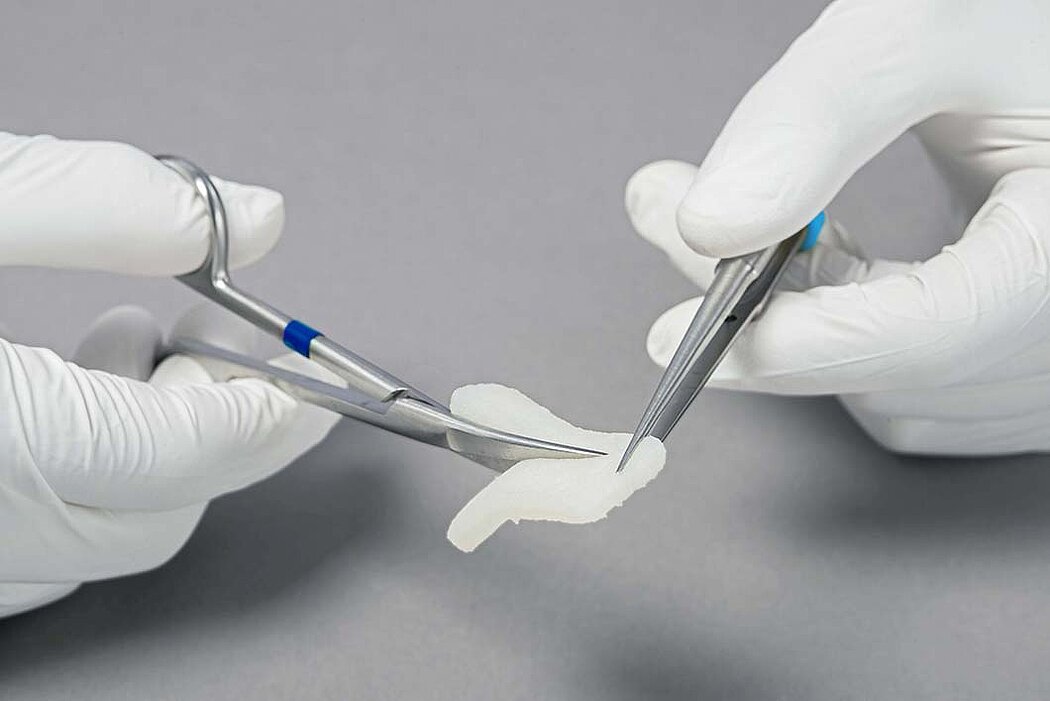
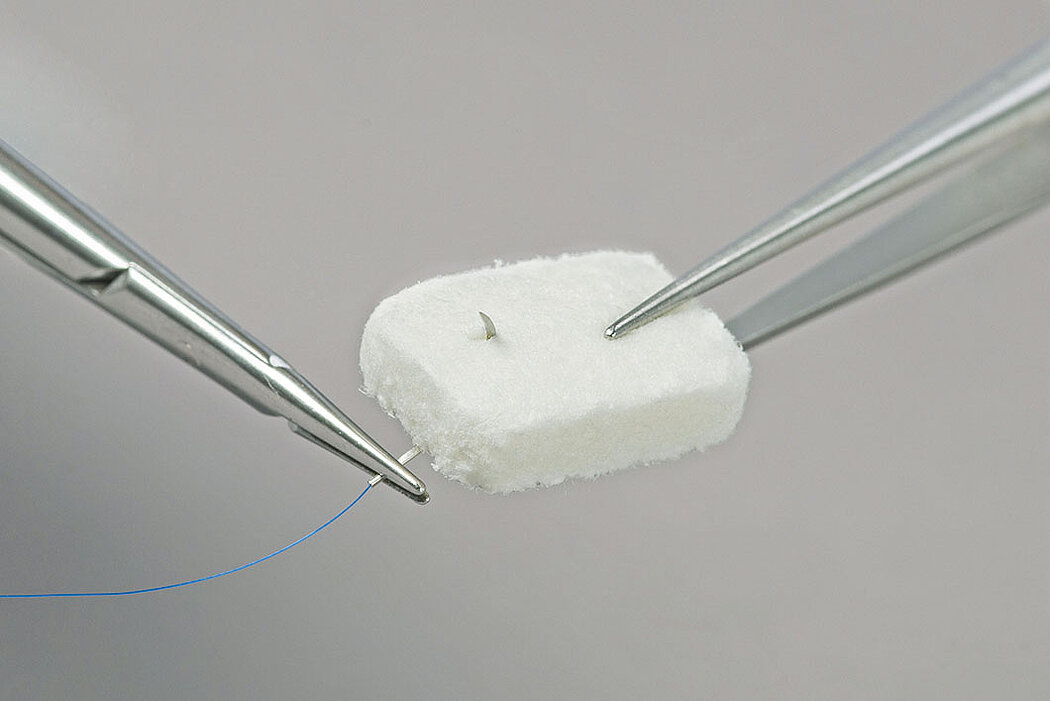
- Geistlich Fibro-Gide® can easily be sutured.1
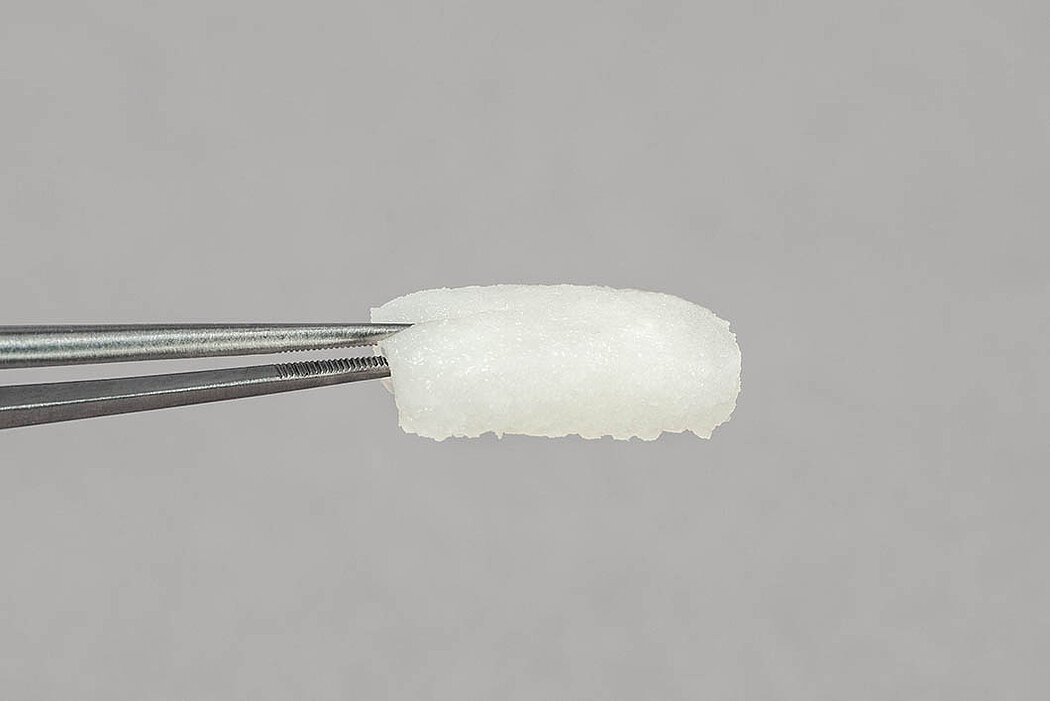
- No need for pre-treatment: Excellent hydrophilic characteristics lead to rapid hydration by patient’s own blood and/or sterile saline solution with a volume increase of approx. 3-12 %.1
- The soaked matrix adapts to contours and adheres well to the defect.1
- Squeezing and re-hydration: Once squeezed, the matrix goes back to its initial state after remoistening (considering the additional swelling of approx. 25 %).1
- Geistlich Fibro-Gide® does not require a second surgical site.2-4
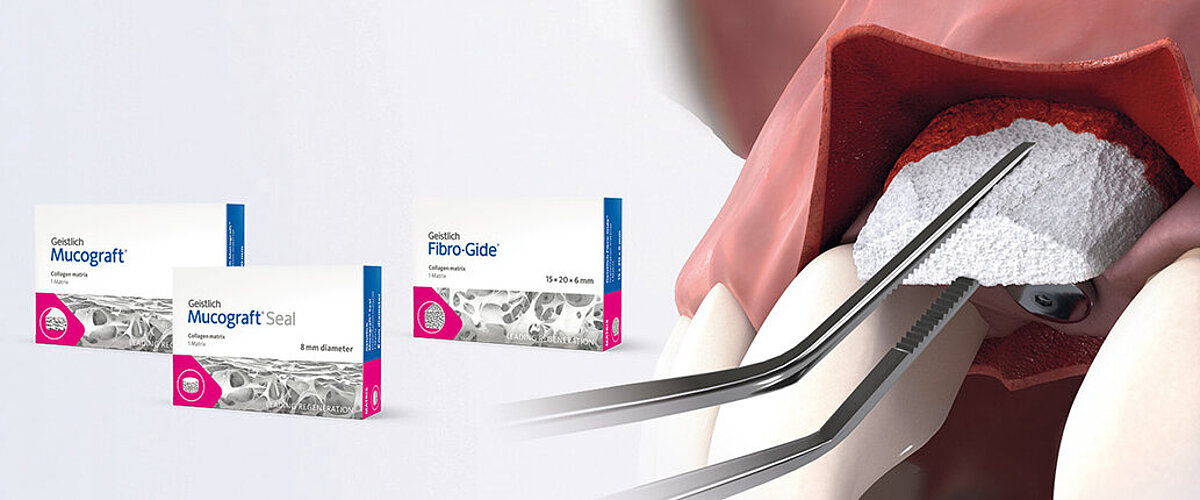
- Geistlich Fibro-Gide® should be used as a submerged graft to increase soft-tissue thickness around implants and natural teeth.1
References
- Data on file. Geistlich Pharma AG, Wolhusen, Switzerland.
- Thoma DS. et al. J Clin Periodontol. 2016 Oct; 43(10): 874–85.
- Del Pizzo M. et al. J Clin Periodontol. 2002 Sep; 29(9): 848–54.
- Soileau KM. & Brannon RB. J Periodontol. 2006 Jul; 77(7): 1267–73.
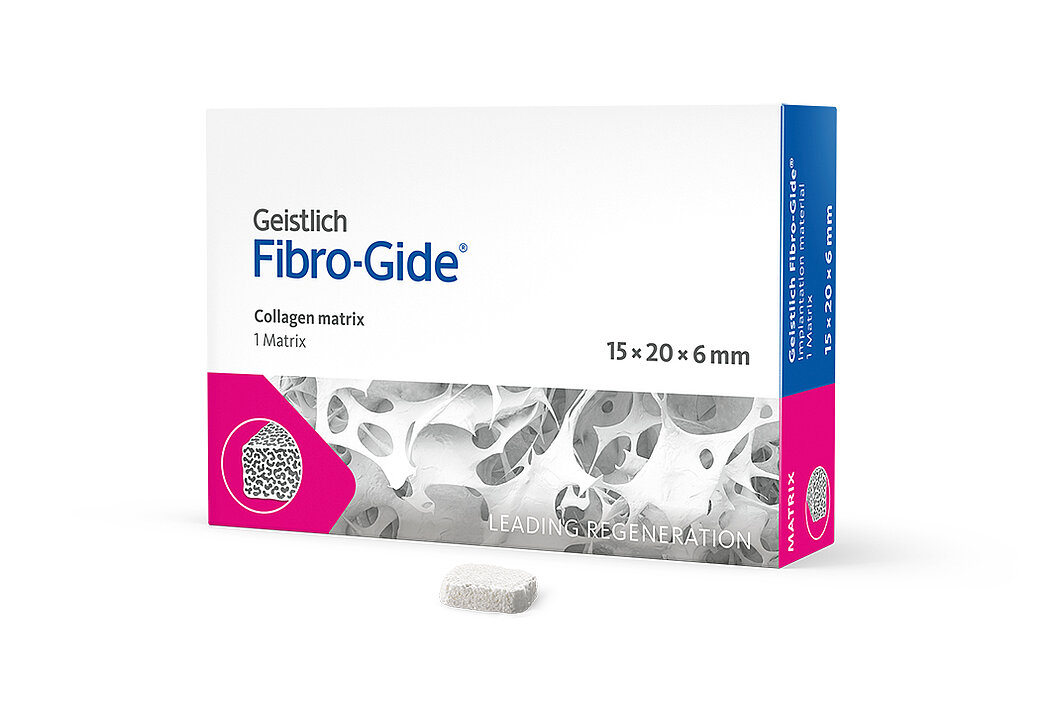
Product Range
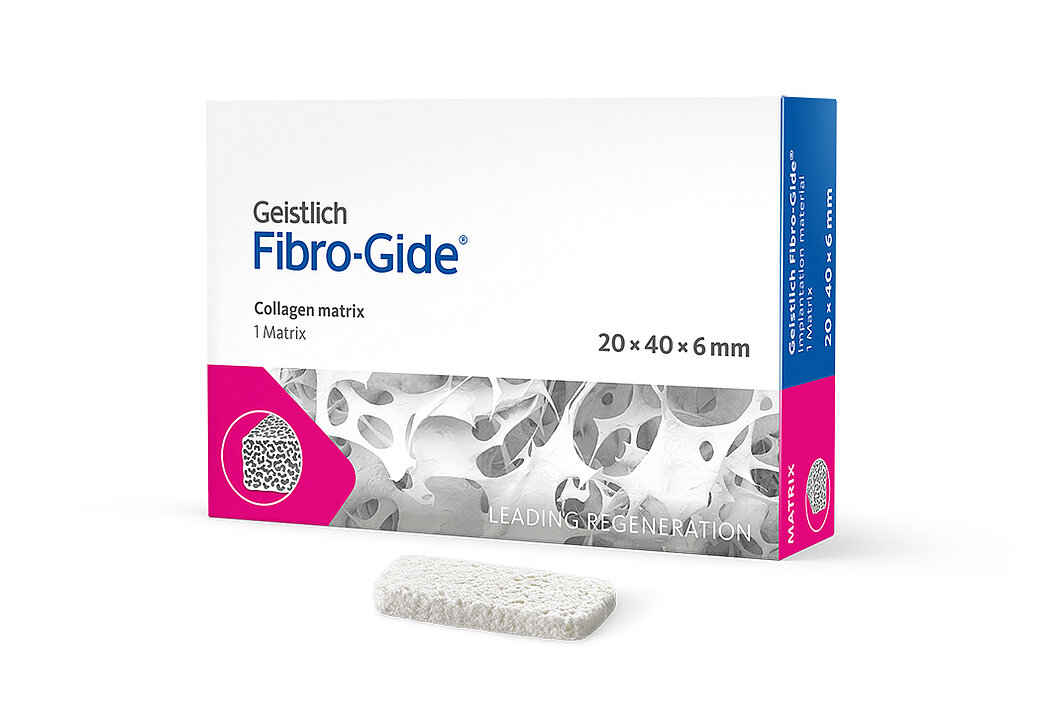
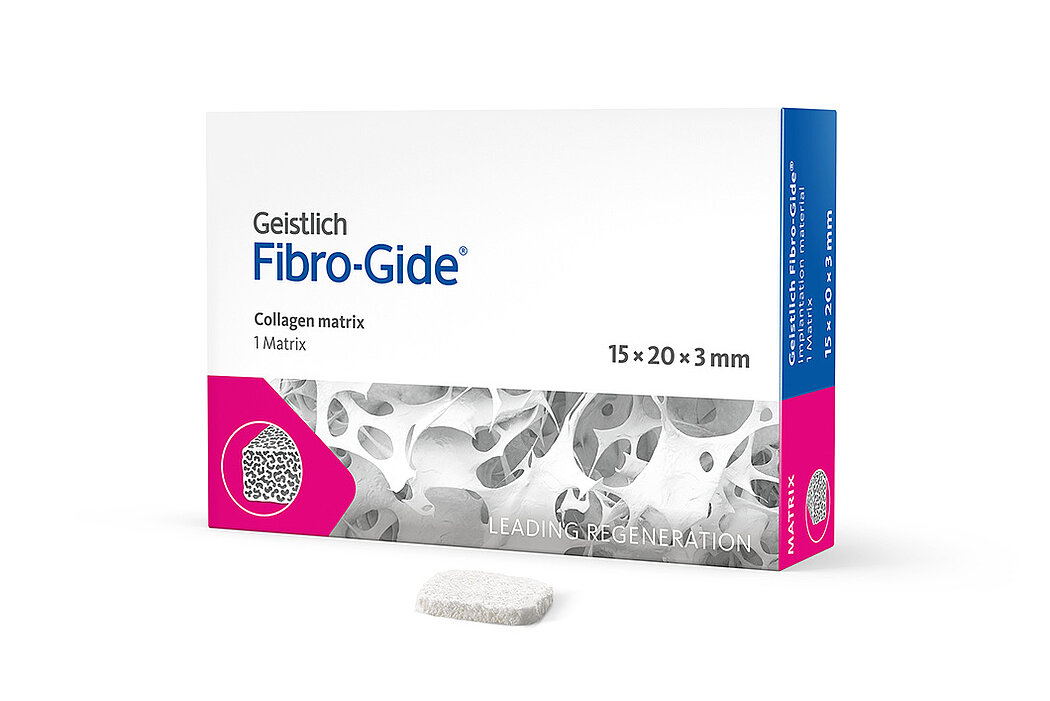
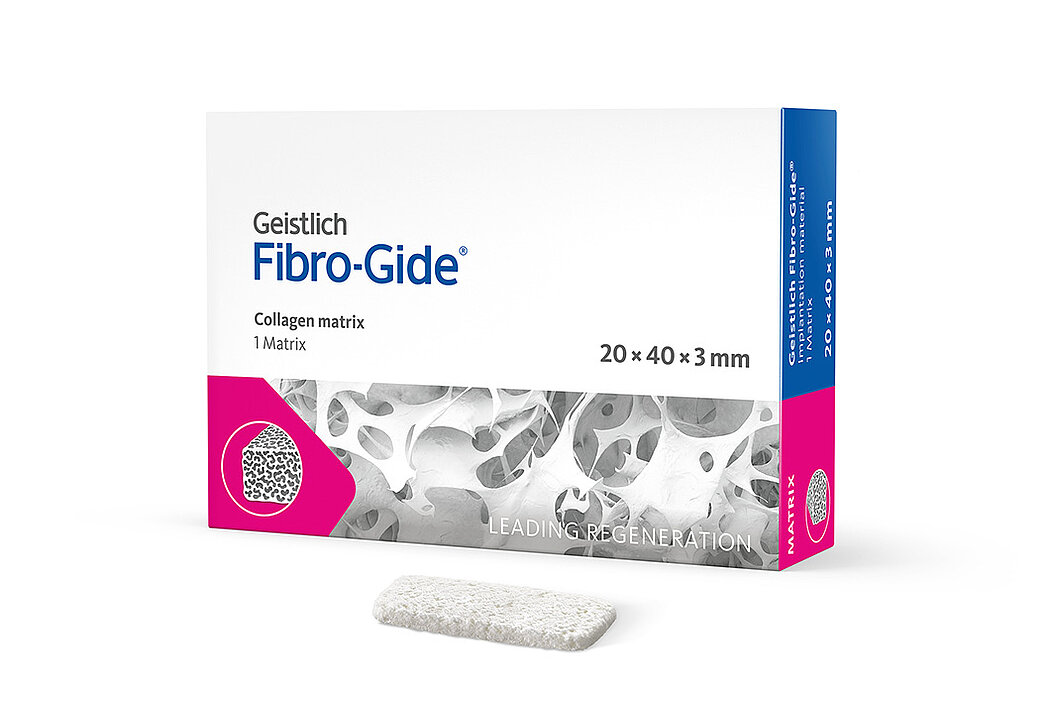
Geistlich Fibro-Gide® is a porcine, porous, resorbable and volume-stable collagen matrix, specifically designed for soft-tissue regeneration.1 The collagen matrix is made of reconstituted collagen and undergoes smart chemically cross-linking to improve its volume stability while maintaining good biocompatibility.1-3 The matrix is available in four sizes:
- 15 x 20 x 6 mm
- 20 x 40 x 6 mm
- 15 x 20 x 3 mm
- 20 x 40 x 3 mm
Product availability may vary from country to country.
Not all products presented on this website are registered and approved for sale and usage in all countries or regions by the relevant authorities. Indications of use may also vary by country and region. Please contact your country representative of Geistlich Pharma AG for product availability and information.